Top 8 List Of Pharmaceutical Companies In Usa List and Guide: How…
Introduction: Navigating the Global Market for list of pharmaceutical companies in usa
In today’s interconnected global marketplace, sourcing pharmaceutical products from reputable companies in the USA presents unique challenges for international B2B buyers. Understanding the landscape of pharmaceutical companies in the USA is essential for ensuring access to high-quality medications, innovative therapies, and compliance with regulatory standards. This guide provides a comprehensive overview of the leading pharmaceutical companies operating within the U.S., including insights into their product offerings, market presence, and specific applications across various therapeutic areas.
The scope of this guide extends beyond mere listings; it encompasses crucial aspects such as supplier vetting processes, cost considerations, and strategies for effective partnership development. By offering a detailed examination of the U.S. pharmaceutical sector, this resource empowers buyers from Africa, South America, the Middle East, and Europe—specifically regions like Saudi Arabia and Brazil—to make informed purchasing decisions that align with their operational needs and regulatory requirements.
Navigating the complexities of sourcing pharmaceutical products can be daunting, but with this guide, stakeholders will gain the necessary insights to identify reliable suppliers, assess product viability, and negotiate favorable terms. Ultimately, this resource serves as a strategic tool to enhance your procurement process, ensuring that you secure the best possible pharmaceutical solutions for your market.
Top 10 List Of Pharmaceutical Companies In Usa Manufacturers & Suppliers List
1. Pharmaceutical Companies – Corporate Information
Domain: drugs.com
Registered: 1998 (27 years)
Introduction: This page contains corporate information for pharmaceutical companies marketing products in the United States. Information includes company addresses, telephone numbers, stock quotes, links to corporate websites, lists of medicines, support and employment opportunities where applicable. Medications listed for each manufacturer or distributor may also be marketed under different names in other coun…
2. Pharmaceutical Companies – Key Players
3. RxList – Comprehensive Pharma Directory
4. Pharmaceutical Giants – Market Capitalization Insights
Domain: companiesmarketcap.com
Registered: 2020 (5 years)
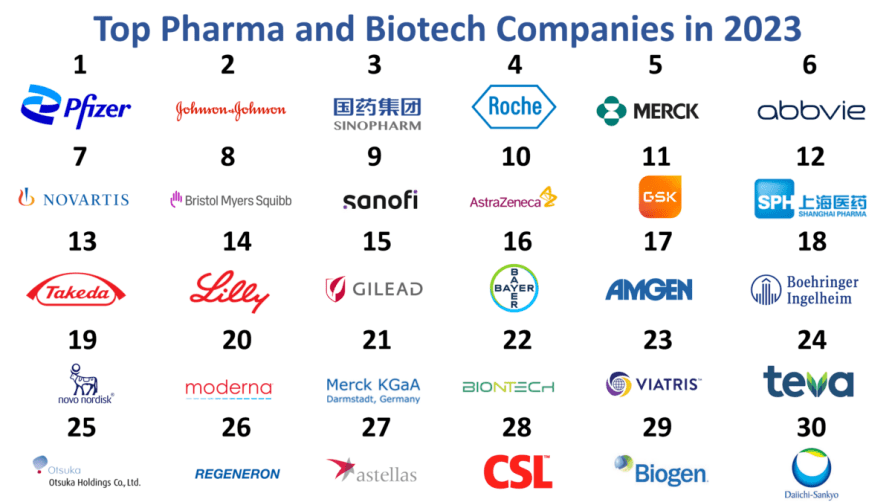
Introduction: Largest pharmaceutical companies by market cap include Eli Lilly, Johnson & Johnson, AbbVie, Novo Nordisk, Roche, AstraZeneca, Novartis, Merck, Amgen, and Pfizer, among others. The list provides their market capitalization, stock prices, and country of origin.
5. Pharmaceutical Giants – Key Players
6. Eli Lilly – Pharmaceuticals
7. Lusha – Sales Intelligence Platform
Domain: lusha.com
Registered: 1999 (26 years)
Introduction: Lusha offers a sales intelligence platform that includes features such as:
– Company and contact data for pharmaceutical manufacturing companies in the United States (6402 companies listed)
– Lead capture on LinkedIn
– Buyer intent tracking to identify in-market buyers
– AI recommendations for daily matched leads
– Automated and personalized email outreach
– Data enrichment to keep CRM updated
– …
8. McKesson – Wholesale Medical Supplies & Healthcare Solutions
Understanding list of pharmaceutical companies in usa Types and Variations
| Type Name | Key Distinguishing Features | Primary B2B Applications | Brief Pros & Cons for Buyers |
|---|---|---|---|
| Big Pharma | Large multinational companies with diverse portfolios | Bulk purchasing, partnership opportunities | Pros: Established brands, extensive R&D. Cons: Higher prices, less flexibility. |
| Generic Manufacturers | Companies producing off-patent drugs at lower costs | Cost-effective sourcing, competitive pricing | Pros: Lower costs, broader accessibility. Cons: Potential quality variability. |
| Biopharmaceuticals | Focus on biologics and advanced therapies | Specialized sourcing for innovative treatments | Pros: Cutting-edge solutions, high efficacy. Cons: Higher development costs, lengthy approval processes. |
| Contract Research Organizations (CROs) | Provide outsourced research and development services | Collaboration for drug development and trials | Pros: Expertise, reduced time to market. Cons: Dependence on third-party performance. |
| Specialty Pharma | Focus on niche markets and complex conditions | Targeted therapies for specific diseases | Pros: Tailored solutions, often unique products. Cons: Limited product range, higher costs. |
What Are the Key Characteristics of Big Pharma Companies?
Big Pharma refers to large, established pharmaceutical companies that have a significant presence in the global market. These companies typically have extensive research and development capabilities and a diverse portfolio of products, including prescription medications, vaccines, and consumer health products. They are often involved in multiple therapeutic areas and have the resources to conduct large-scale clinical trials. For B2B buyers, engaging with Big Pharma can provide access to established brands and innovative products, although the pricing may be higher compared to smaller firms.
How Do Generic Manufacturers Differ from Other Pharmaceutical Companies?
Generic manufacturers produce medications that are chemically identical to brand-name drugs whose patents have expired. These companies focus on cost-effective production, allowing them to offer lower-priced alternatives to the market. B2B buyers often turn to generic manufacturers for bulk purchasing to reduce overall costs. While the affordability of generics is a significant advantage, buyers should be aware of potential variations in quality and availability.
What Are the Unique Features of Biopharmaceutical Companies?
Biopharmaceutical companies specialize in developing drugs derived from biological sources, such as proteins and antibodies. These firms are at the forefront of innovation, focusing on advanced therapies for complex diseases. B2B buyers seeking cutting-edge solutions may find biopharmaceuticals particularly appealing. However, the development and approval processes for these products can be lengthy and costly, which may affect pricing and availability.
Why Consider Contract Research Organizations (CROs) for B2B Collaborations?
CROs provide outsourced services for clinical trials and research, allowing pharmaceutical companies to leverage external expertise in drug development. They play a crucial role in facilitating faster market entry for new products. B2B buyers can benefit from collaborations with CROs by accessing specialized knowledge and resources. However, reliance on these organizations can introduce risks related to performance and communication.
What Are the Advantages of Specialty Pharma Companies?
Specialty pharmaceutical companies focus on niche markets, often developing treatments for rare or complex conditions. These firms may provide tailored solutions that address specific patient needs. B2B buyers may find value in partnering with specialty pharma for unique products that are not widely available. However, the limited product range and higher costs associated with specialty medications can be potential drawbacks for buyers looking for broader options.
Key Industrial Applications of list of pharmaceutical companies in usa
| Industry/Sector | Specific Application of list of pharmaceutical companies in usa | Value/Benefit for the Business | Key Sourcing Considerations for this Application |
|---|---|---|---|
| Healthcare | Sourcing innovative pharmaceuticals for chronic diseases | Access to cutting-edge treatments enhances patient care outcomes | Regulatory compliance, quality assurance, and supply chain reliability |
| Biotechnology | Collaborating on drug development and clinical trials | Accelerated time-to-market for new therapies | Intellectual property considerations, partnership agreements, and funding |
| Generic Pharmaceuticals | Procurement of cost-effective generic drugs | Reduces healthcare costs while maintaining treatment efficacy | Pricing agreements, market access strategies, and regulatory approvals |
| Medical Devices | Integration of pharmaceuticals with medical devices | Improved patient outcomes through combination therapies | Compatibility with existing systems, supply chain logistics, and quality standards |
| Research and Development (R&D) | Sourcing raw materials for pharmaceutical research | Enhanced innovation capabilities and product development efficiency | Supplier reliability, sourcing of high-quality raw materials, and compliance with R&D standards |
How Can Pharmaceutical Companies in the USA Address Healthcare Needs?
Pharmaceutical companies in the USA play a crucial role in sourcing innovative treatments for chronic diseases such as diabetes, hypertension, and cancer. By partnering with these companies, international B2B buyers can access cutting-edge medications that improve patient outcomes. Key considerations include ensuring regulatory compliance and maintaining high-quality standards throughout the supply chain, particularly for buyers in regions with stringent import regulations.
What Role Does Biotechnology Play in Drug Development?
Biotechnology firms within the pharmaceutical sector are pivotal in drug development and clinical trials. By collaborating with these companies, buyers can expedite the introduction of new therapies to the market. This requires a solid understanding of intellectual property rights and effective partnership agreements. For international buyers, especially in Africa and South America, securing funding and navigating local regulations are essential for successful collaboration.
How Can Generic Pharmaceuticals Lower Healthcare Costs?
The procurement of generic drugs from US pharmaceutical companies offers a cost-effective solution for healthcare providers looking to minimize expenses while ensuring treatment efficacy. Generic medications can significantly reduce healthcare costs in emerging markets. Buyers must focus on pricing agreements and strategies for market access, along with ensuring that the generic products meet local regulatory standards.
What Are the Benefits of Integrating Pharmaceuticals with Medical Devices?
The integration of pharmaceuticals with medical devices is transforming patient care. This synergy allows for combination therapies that enhance treatment effectiveness, particularly in areas like insulin delivery systems for diabetes management. For businesses, key sourcing considerations include ensuring compatibility with existing medical devices and adhering to quality standards to avoid regulatory issues.
How Can Sourcing Raw Materials Enhance R&D Capabilities?
Sourcing high-quality raw materials is vital for pharmaceutical research and development. Companies that prioritize this can enhance their innovation capabilities and streamline product development processes. International buyers must ensure supplier reliability and compliance with R&D standards, particularly in regions where sourcing raw materials can be challenging due to local regulations or supply chain disruptions.
3 Common User Pain Points for ‘list of pharmaceutical companies in usa’ & Their Solutions
Scenario 1: Navigating Regulatory Compliance in International Transactions
The Problem:
For B2B buyers in regions like Africa and South America, sourcing pharmaceutical products from U.S. companies often involves navigating a complex web of regulatory requirements. Each country has its own rules regarding the importation of pharmaceuticals, including licensing, documentation, and adherence to safety standards. Buyers may find themselves facing delays or penalties due to a lack of understanding of these regulations, which can jeopardize not only their operational timelines but also their business relationships with suppliers.
The Solution:
To effectively navigate these regulatory challenges, B2B buyers should invest time in researching the specific import regulations of their home country concerning pharmaceutical products. They should also leverage the resources available on the U.S. pharmaceutical companies’ websites, which often contain sections dedicated to international business. Engaging with legal or compliance experts who specialize in pharmaceutical imports can provide additional insights and mitigate risks. Furthermore, establishing clear communication with U.S. suppliers about regulatory expectations can facilitate smoother transactions and enhance trust between parties.
Scenario 2: Ensuring Product Quality and Authenticity
The Problem:
International buyers often face concerns about product quality and authenticity when sourcing from U.S. pharmaceutical companies. With the prevalence of counterfeit drugs in the global market, buyers need assurance that they are purchasing genuine products. This anxiety can be compounded by the sheer number of companies listed, making it difficult to vet suppliers effectively.
The Solution:
To address these concerns, buyers should prioritize sourcing from well-established pharmaceutical companies with a strong reputation and verifiable track record. Utilizing resources like the Pharmaceutical Company Directory can help identify companies that have been recognized for their quality and compliance with good manufacturing practices (GMP). Additionally, buyers can request Certificates of Analysis (CoA) and other relevant documentation that demonstrates the quality and authenticity of the products. Conducting site visits or third-party audits of manufacturing facilities can also provide peace of mind regarding product integrity.
Scenario 3: Overcoming Language and Communication Barriers
The Problem:
For B2B buyers from non-English speaking countries, language barriers can pose significant challenges when trying to communicate with U.S. pharmaceutical companies. Misunderstandings can lead to errors in orders, delays in shipments, and even compliance issues. This problem is exacerbated by the technical language often used in pharmaceutical discussions, which may not translate well.
The Solution:
B2B buyers should consider employing professional translation services when dealing with U.S. pharmaceutical companies to ensure that all communications are clear and accurate. Additionally, utilizing bilingual representatives or consultants who are familiar with both the pharmaceutical industry and the specific needs of the buyer’s market can enhance communication. When reaching out to suppliers, buyers should prepare a list of key questions and terms in both languages to facilitate smoother discussions. Furthermore, many U.S. pharmaceutical companies are beginning to offer multilingual support on their websites, so buyers should take advantage of these resources to foster better understanding and collaboration.
Strategic Material Selection Guide for list of pharmaceutical companies in usa
What Are the Key Materials Used in Pharmaceutical Manufacturing?
When selecting materials for pharmaceutical applications, it is crucial to consider properties that ensure product performance, safety, and regulatory compliance. Here, we analyze four common materials used by pharmaceutical companies in the USA, focusing on their properties, advantages, disadvantages, and considerations for international buyers.
1. Stainless Steel
Key Properties:
Stainless steel is known for its excellent corrosion resistance, high strength, and ability to withstand elevated temperatures and pressures. It is often used in equipment such as tanks, piping, and valves.
Pros & Cons:
Stainless steel is durable and easy to clean, making it suitable for sterile environments. However, it can be expensive compared to other materials and may require complex manufacturing processes, including welding and polishing.
Impact on Application:
Stainless steel is compatible with a wide range of media, including corrosive substances. Its non-reactive nature ensures that it does not contaminate the pharmaceutical products.
Considerations for International Buyers:
Buyers from regions like Africa and the Middle East should ensure compliance with international standards such as ASTM and ISO. Additionally, understanding local regulations regarding material sourcing and manufacturing practices is essential.
2. Glass
Key Properties:
Glass is non-reactive, transparent, and impermeable, making it ideal for packaging pharmaceuticals. It can withstand high temperatures and is resistant to many chemicals.
Pros & Cons:
The primary advantage of glass is its inertness, preventing chemical interactions with the contents. However, it is fragile and can break easily, leading to potential safety hazards and increased shipping costs.
Impact on Application:
Glass is often used for vials, ampoules, and syringes, ensuring product integrity and sterility. Its compatibility with various media makes it a preferred choice for sensitive formulations.
Considerations for International Buyers:
Compliance with packaging regulations, such as those set by the FDA and EMA, is critical. Buyers should also consider the environmental impact of glass recycling in their regions.
3. Polypropylene
Key Properties:
Polypropylene is a thermoplastic polymer known for its chemical resistance, low density, and ability to withstand moderate temperatures. It is often used in containers and closures.
Pros & Cons:
This material is lightweight and cost-effective, making it suitable for large-scale production. However, it has lower temperature resistance compared to metals and glass, which may limit its application in certain processes.
Impact on Application:
Polypropylene is compatible with a wide range of chemicals, making it ideal for packaging and storage. However, it may not be suitable for high-temperature sterilization processes.
Considerations for International Buyers:
Buyers should verify compliance with material safety standards and regulations in their countries. Understanding local preferences for sustainable materials can also influence purchasing decisions.
4. Silicone
Key Properties:
Silicone is a synthetic polymer known for its flexibility, thermal stability, and resistance to extreme temperatures. It is commonly used in seals, gaskets, and tubing.
Pros & Cons:
Silicone offers excellent biocompatibility, making it suitable for medical devices and drug delivery systems. However, it can be more expensive than other polymers and may require specialized manufacturing techniques.
Impact on Application:
Silicone’s flexibility and resistance to degradation make it ideal for applications involving contact with biological materials. Its compatibility with various media ensures product safety.
Considerations for International Buyers:
International buyers should consider regulatory compliance regarding medical-grade silicones and their applications in drug delivery. Familiarity with local sourcing options can also enhance supply chain efficiency.
Summary Table of Material Selection
| Material | Typical Use Case for list of pharmaceutical companies in usa | Key Advantage | Key Disadvantage/Limitation | Relative Cost (Low/Med/High) |
|---|---|---|---|---|
| Stainless Steel | Tanks, piping, valves | Excellent corrosion resistance | High cost and complex manufacturing | High |
| Glass | Vials, ampoules, syringes | Inert and prevents contamination | Fragile and costly to ship | Med |
| Polypropylene | Containers, closures | Lightweight and cost-effective | Lower temperature resistance | Low |
| Silicone | Seals, gaskets, tubing | Excellent biocompatibility | Higher cost and specialized techniques | Med |
This guide serves as a strategic resource for international B2B buyers looking to navigate material selection in the pharmaceutical sector, ensuring they make informed decisions that align with their operational needs and regulatory requirements.
In-depth Look: Manufacturing Processes and Quality Assurance for list of pharmaceutical companies in usa
What Are the Main Stages of Pharmaceutical Manufacturing Processes?
Pharmaceutical manufacturing is a complex and multi-faceted process that involves several critical stages to ensure the production of safe and effective drugs. The primary stages include material preparation, forming, assembly, and finishing.
-
Material Preparation: This initial phase involves the procurement and testing of raw materials to ensure they meet predefined specifications. Materials undergo rigorous quality checks, including identity verification and purity assessments. Suppliers must comply with Good Manufacturing Practices (GMP) to guarantee that only high-quality components are used in production.
-
Forming: In this stage, the raw materials are transformed into the desired pharmaceutical forms, such as tablets, capsules, or injectables. Techniques employed can vary widely, including granulation, milling, and lyophilization. For instance, in tablet manufacturing, active pharmaceutical ingredients (APIs) are blended with excipients before being compressed into tablets.
-
Assembly: This phase involves the packaging of the formed products. The assembly line must maintain sterility, especially for injectable products. Automation is commonly used to enhance efficiency and accuracy during this stage, which often includes bottling, labeling, and secondary packaging.
-
Finishing: The final stage includes quality control checks, storage, and distribution preparations. Products are subjected to final inspections to ensure they meet regulatory standards before being released to the market.
What Key Techniques Are Employed in Pharmaceutical Manufacturing?
Pharmaceutical companies employ several advanced techniques to optimize their manufacturing processes. These include:
-
Continuous Manufacturing: This modern approach allows for uninterrupted production, enhancing efficiency and reducing waste. Continuous processes can lead to better control of the quality and consistency of the final product.
-
Process Analytical Technology (PAT): PAT tools enable real-time monitoring and control of the manufacturing process, allowing manufacturers to make immediate adjustments to maintain quality.
-
Automation and Robotics: Automation minimizes human error and increases production speed, particularly in high-volume settings. Robotics play a crucial role in tasks such as filling, capping, and labeling.
-
3D Printing: This emerging technology is being explored for personalized medicine, allowing for the production of patient-specific dosages and formulations.
How Is Quality Assurance Implemented in Pharmaceutical Companies?
Quality assurance (QA) is a cornerstone of pharmaceutical manufacturing, ensuring that products are consistently produced and controlled according to quality standards. Companies typically adhere to both international and industry-specific quality standards.
-
International Standards: ISO 9001 is a widely recognized quality management system standard that emphasizes continuous improvement and customer satisfaction. Adopting this standard helps companies streamline their processes and enhance product quality.
-
Industry-Specific Standards: Compliance with regulations such as the European Union’s CE mark for medical devices, and adherence to the principles of Good Manufacturing Practices (GMP) are crucial for pharmaceutical companies. These regulations ensure that products are safe, effective, and of high quality.
What Are the Key Quality Control Checkpoints in Pharmaceutical Manufacturing?
Quality control (QC) is an integral part of the manufacturing process, involving multiple checkpoints to ensure product integrity:
-
Incoming Quality Control (IQC): This checkpoint involves testing raw materials before they enter production. Materials are assessed for identity, purity, and quality to prevent defects in the final product.
-
In-Process Quality Control (IPQC): During manufacturing, ongoing assessments are conducted to ensure processes are within specified parameters. This includes monitoring critical process variables and conducting in-process tests.
-
Final Quality Control (FQC): After manufacturing, the final product undergoes comprehensive testing to verify that it meets all specifications and regulatory requirements before it is released to the market.
What Testing Methods Are Commonly Used in Pharmaceutical Quality Control?
A variety of testing methods are employed in pharmaceutical quality control to ensure product safety and efficacy:
-
Chemical Analysis: Techniques such as High-Performance Liquid Chromatography (HPLC) and Mass Spectrometry (MS) are used to determine the concentration and purity of active ingredients.
-
Microbiological Testing: This testing ensures that products are free from harmful microorganisms. Methods include sterility tests and bioburden testing.
-
Stability Testing: This assesses how the quality of a pharmaceutical product varies with time under the influence of environmental factors such as temperature and humidity.
How Can B2B Buyers Verify Supplier Quality Control Practices?
For international B2B buyers, verifying a supplier’s quality control practices is essential for ensuring product reliability. Here are several strategies to consider:
-
Supplier Audits: Conducting on-site audits of suppliers allows buyers to assess their manufacturing processes and quality control systems firsthand. This includes reviewing documentation, observing practices, and interviewing staff.
-
Quality Control Reports: Requesting detailed QC reports, including testing results and compliance certifications, provides insight into a supplier’s quality assurance practices.
-
Third-Party Inspections: Engaging independent third-party organizations to conduct inspections can provide an unbiased evaluation of a supplier’s quality control measures.
What Are the Quality Control and Certification Nuances for International B2B Buyers?
B2B buyers from regions such as Africa, South America, the Middle East, and Europe should be aware of specific nuances when dealing with pharmaceutical suppliers:
-
Regulatory Compliance: Different regions may have varying regulatory requirements. Buyers should ensure that suppliers comply with local regulations and international standards relevant to their markets.
-
Documentation and Certifications: Buyers should verify that suppliers possess necessary certifications such as ISO 9001, GMP, and any local certifications required by their respective countries.
-
Cultural and Communication Barriers: Understanding the cultural context and communication styles of suppliers can facilitate smoother interactions and negotiations. Establishing clear expectations and open lines of communication is crucial for successful partnerships.
By comprehensively understanding the manufacturing processes and quality assurance protocols utilized by pharmaceutical companies in the USA, international B2B buyers can make informed decisions and establish reliable supply chains. This knowledge not only enhances product safety and efficacy but also builds trust in supplier relationships across borders.
Practical Sourcing Guide: A Step-by-Step Checklist for ‘list of pharmaceutical companies in usa’
Introduction
This guide serves as a practical checklist for international B2B buyers seeking to procure products from pharmaceutical companies in the USA. By following these steps, you can ensure a comprehensive evaluation of potential suppliers, helping you make informed decisions that align with your business needs.
Step 1: Identify Your Specific Needs
Clearly define what products or services you require from pharmaceutical companies. This includes understanding the therapeutic areas, dosage forms, and regulatory requirements relevant to your market. By pinpointing your needs, you can streamline your search and engage with companies that specialize in your required sectors.
- Therapeutic Areas: Consider whether you need medications for chronic diseases, acute conditions, or specialty pharmaceuticals.
- Regulatory Compliance: Ensure that the products meet the regulations of your home country to avoid compliance issues.
Step 2: Conduct Market Research
Research the pharmaceutical landscape in the USA to identify potential suppliers. Utilize online directories and industry reports to gather data on the most reputable companies, their product offerings, and market presence.
- Use Resources: Websites like Drugs.com and GlobalData can provide extensive lists and insights on pharmaceutical companies.
- Market Trends: Stay updated on current trends that may affect product availability, such as new drug approvals or emerging technologies.
Step 3: Evaluate Potential Suppliers
Before making commitments, thoroughly vet potential suppliers. This involves reviewing their company profiles, product portfolios, and track records in the industry.
- Company Background: Look for information on their history, market position, and any awards or recognitions.
- References and Case Studies: Request testimonials from existing clients, especially those in similar markets, to gauge reliability and service quality.
Step 4: Verify Certifications and Compliance
Ensure that the companies you are considering have the necessary certifications and comply with industry standards. This can include FDA approvals, GMP (Good Manufacturing Practices) certifications, and ISO standards.
- Quality Assurance: Confirm that their manufacturing processes meet rigorous quality standards to mitigate risks associated with product efficacy and safety.
- Regulatory Adherence: Check if they have a history of compliance issues, which could indicate potential risks in partnership.
Step 5: Assess Financial Stability
Evaluate the financial health of potential suppliers to ensure they can meet your long-term needs. Companies with strong financials are more likely to invest in innovation and maintain stable operations.
- Market Capitalization: Review their market cap and revenue trends to understand their position in the industry.
- Credit Ratings: Consider obtaining credit reports or assessments from financial institutions to gain insights into their creditworthiness.
Step 6: Negotiate Terms and Conditions
Once you have shortlisted potential suppliers, initiate discussions to negotiate terms of supply, pricing, and delivery schedules. Clear agreements will help establish a solid foundation for the partnership.
- Flexibility and Scalability: Discuss options for scaling orders up or down based on your business needs.
- Payment Terms: Ensure that payment terms are clear and favorable to your cash flow requirements.
Step 7: Establish a Communication Plan
Develop a clear communication strategy with your chosen suppliers. Effective communication can prevent misunderstandings and foster strong relationships.
- Regular Updates: Set expectations for regular updates on order status, product changes, and any relevant market developments.
- Point of Contact: Designate a primary contact person from both sides to facilitate smooth interactions.
By following these steps, you can confidently navigate the process of sourcing pharmaceutical companies in the USA, ensuring you find the right partners to support your business objectives.
Comprehensive Cost and Pricing Analysis for list of pharmaceutical companies in usa Sourcing
What Are the Key Cost Components in Pharmaceutical Sourcing from US Companies?
When sourcing from US pharmaceutical companies, understanding the cost structure is crucial for international B2B buyers. The primary cost components include:
-
Materials: The cost of raw materials can vary significantly based on the type of pharmaceuticals being produced, whether they are active pharmaceutical ingredients (APIs) or finished products. Sourcing high-quality materials from reputable suppliers is essential, as it directly impacts product quality and compliance with regulatory standards.
-
Labor: Labor costs in the US can be high due to stringent labor laws and the need for skilled professionals in research and manufacturing. This is particularly relevant for companies engaged in complex drug formulations or biopharmaceuticals.
-
Manufacturing Overhead: This includes utilities, equipment maintenance, and facility costs. US pharmaceutical companies often invest heavily in state-of-the-art facilities to ensure compliance with FDA regulations, which can inflate overhead costs.
-
Tooling: Specialized tooling for production lines can be a significant upfront investment. Companies may pass these costs onto buyers, especially for customized products that require unique manufacturing processes.
-
Quality Control (QC): Rigorous QC measures are mandatory in the pharmaceutical industry to ensure product safety and efficacy. The costs associated with these processes can vary but are critical to maintaining certifications and regulatory compliance.
-
Logistics: Transportation and warehousing costs can be substantial, particularly for temperature-sensitive products. Buyers should consider the shipping methods and associated costs when evaluating total procurement expenses.
-
Margin: Pharmaceutical companies typically operate with a significant markup to cover research and development costs, regulatory compliance, and profit margins. This markup can vary based on the company’s market position and the competitive landscape.
How Do Price Influencers Affect Pharmaceutical Product Costs?
Several factors influence pricing in the pharmaceutical sector, which international buyers must consider:
-
Volume/MOQ: Minimum order quantities (MOQs) can impact pricing significantly. Larger orders often result in lower per-unit costs, making it advantageous for buyers to negotiate bulk purchasing agreements.
-
Specifications and Customization: Customized products or formulations may incur additional costs due to the need for specialized manufacturing processes. Buyers should clarify their requirements early to avoid unexpected expenses.
-
Materials: The choice of materials can greatly influence costs. Premium or specialized materials will increase the overall price, so it’s essential to balance quality with cost.
-
Quality and Certifications: Products with higher regulatory certifications or quality assurances typically command higher prices. Buyers should weigh the benefits of these certifications against their budget constraints.
-
Supplier Factors: The reputation and reliability of the supplier can also affect pricing. Established companies with a strong track record may charge a premium, but this can be justified by reduced risk and higher quality assurance.
-
Incoterms: Understanding Incoterms is vital for international transactions, as they define the responsibilities of buyers and sellers in shipping. The choice of Incoterms can affect the total cost of ownership, including shipping, insurance, and customs duties.
What Tips Can Help International Buyers Optimize Pharmaceutical Procurement Costs?
To navigate the complexities of pharmaceutical sourcing effectively, buyers should consider the following strategies:
-
Negotiation: Engage in open discussions with suppliers about pricing, terms, and potential discounts. Building a good relationship can lead to more favorable terms and conditions.
-
Cost-Efficiency: Analyze total costs beyond the initial purchase price. Consider logistics, storage, and potential wastage to determine the most cost-effective sourcing strategy.
-
Total Cost of Ownership (TCO): Assess the TCO for products, including all associated costs from procurement to delivery. This holistic view can help identify the best value offerings.
-
Pricing Nuances: Be aware that pharmaceutical pricing can vary significantly based on market dynamics, exchange rates, and geopolitical factors. This is particularly relevant for buyers from Africa, South America, the Middle East, and Europe, who may face additional import duties and regulatory requirements.
Disclaimer
The prices mentioned in this analysis are indicative and can vary based on market conditions, supplier negotiations, and specific buyer requirements. Always conduct thorough due diligence before making procurement decisions.
Alternatives Analysis: Comparing list of pharmaceutical companies in usa With Other Solutions
Exploring Alternative Solutions to the List of Pharmaceutical Companies in the USA
In the dynamic landscape of the pharmaceutical industry, B2B buyers often seek comprehensive resources to make informed decisions about partnerships, sourcing, and product development. While a list of pharmaceutical companies in the USA serves as a valuable tool for identifying potential collaborators, there are alternative methods and technologies available that can achieve similar goals. Below, we compare the traditional list with two viable alternatives: pharmaceutical databases and industry networking platforms.
| Comparison Aspect | List of Pharmaceutical Companies in USA | Pharmaceutical Databases | Industry Networking Platforms |
|---|---|---|---|
| Performance | Provides a static reference point | Offers real-time updates and analytics | Facilitates direct interactions and collaborations |
| Cost | Generally free or low-cost | Subscription-based, can be high | Free or low-cost, depending on features |
| Ease of Implementation | Simple to access and use | Requires training for effective use | User-friendly, but may require registration |
| Maintenance | Minimal upkeep required | Regular updates needed | Continuous engagement necessary |
| Best Use Case | Quick reference for company info | In-depth research and analysis | Building relationships and partnerships |
What Are the Advantages and Disadvantages of Pharmaceutical Databases?
Pharmaceutical databases, such as DrugBank or PharmaCircle, provide comprehensive, real-time information about drug formulations, clinical trials, and market analytics. Pros include the ability to access detailed data on drug interactions, regulatory approvals, and competitive landscapes. This can greatly enhance research capabilities and strategic planning. However, the cons often involve subscription costs, which can be prohibitive for smaller companies or startups. Additionally, users may require training to navigate the extensive features and functionalities effectively.
How Do Industry Networking Platforms Enhance B2B Opportunities?
Industry networking platforms, such as LinkedIn or specialized forums like BioPharma Dive, allow B2B buyers to connect directly with pharmaceutical professionals, facilitating discussions, partnerships, and collaborations. Pros include the ability to build relationships and gain insights directly from industry experts, leading to potential business opportunities. These platforms often provide a community-driven environment for knowledge sharing. However, the cons might involve the need for consistent engagement and the challenge of filtering valuable information from noise in a crowded space.
How Can B2B Buyers Choose the Right Solution for Their Needs?
When selecting the right solution, B2B buyers must assess their specific needs and objectives. If the priority is quick access to company information and product details, a list of pharmaceutical companies in the USA may suffice. However, if in-depth market analysis or real-time data is required, investing in a pharmaceutical database could be advantageous. Alternatively, for those focused on building relationships and partnerships, industry networking platforms offer invaluable opportunities for engagement. Ultimately, the best choice depends on the buyer’s strategic goals, budget constraints, and the desired depth of information.
Essential Technical Properties and Trade Terminology for list of pharmaceutical companies in usa
What Are the Key Technical Properties in the Pharmaceutical Industry?
When engaging with pharmaceutical companies in the USA, understanding critical technical properties is essential for ensuring compliance and quality in products. Here are some of the vital specifications that B2B buyers should be aware of:
-
Active Pharmaceutical Ingredient (API) Quality
The quality of APIs is paramount, as they determine the efficacy and safety of the final product. Buyers should look for APIs that meet stringent regulatory standards set by the FDA or EMA, ensuring consistency and reliability in medication. APIs are often subjected to rigorous testing for purity, potency, and stability. -
Formulation Type
Pharmaceuticals come in various forms, such as tablets, capsules, injectables, and topical applications. Each formulation type has specific manufacturing requirements and stability considerations. Understanding the formulation helps buyers choose the right products for their market needs, affecting absorption rates and patient compliance. -
Stability and Shelf Life
The stability of pharmaceutical products affects their shelf life and efficacy. Buyers must consider the stability data provided by manufacturers, which includes storage conditions, expiration dates, and degradation rates. Products with longer shelf lives reduce the frequency of reordering and stock management issues. -
Manufacturing Processes
Different manufacturing processes (e.g., sterile vs. non-sterile production) can impact product quality and compliance. Understanding these processes helps buyers assess the capabilities of pharmaceutical companies and their adherence to Good Manufacturing Practices (GMP). This knowledge is crucial when evaluating potential suppliers. -
Regulatory Compliance
Compliance with local and international regulations is non-negotiable in the pharmaceutical industry. Buyers should ensure that manufacturers are compliant with FDA regulations, as well as any relevant international standards, which can affect market access and product acceptance.
What Are Common Trade Terms Used in the Pharmaceutical Sector?
Navigating the pharmaceutical landscape requires familiarity with specific trade terminology. Here are several key terms that B2B buyers should know:
-
OEM (Original Equipment Manufacturer)
In the pharmaceutical context, OEM refers to companies that produce products or components that are then rebranded and sold by other companies. Understanding OEM relationships can help buyers identify potential partners for sourcing products that meet their specific branding requirements. -
MOQ (Minimum Order Quantity)
This term indicates the smallest quantity of a product that a supplier is willing to sell. Knowing the MOQ is crucial for buyers to manage inventory and cash flow effectively. It can also affect pricing, as larger orders often lead to discounts. -
RFQ (Request for Quotation)
An RFQ is a formal process where buyers request pricing and terms from suppliers. This document outlines the specifications and quantities needed, enabling suppliers to provide accurate quotes. Using an RFQ can streamline the purchasing process and ensure competitive pricing. -
Incoterms (International Commercial Terms)
These are standardized trade terms that define the responsibilities of buyers and sellers in international transactions. Familiarity with Incoterms is essential for understanding shipping costs, risk transfer, and delivery obligations, which can significantly impact overall procurement strategies. -
CTD (Common Technical Document)
The CTD is a set of specifications for submitting applications to regulatory authorities. Understanding the CTD framework is essential for buyers involved in sourcing or developing pharmaceutical products, as it ensures compliance and facilitates smoother regulatory interactions.
By grasping these technical properties and trade terms, B2B buyers can better navigate the pharmaceutical landscape, leading to informed decisions and successful partnerships with US pharmaceutical companies.
Navigating Market Dynamics and Sourcing Trends in the list of pharmaceutical companies in usa Sector
What Are the Current Market Dynamics and Key Trends in the Pharmaceutical Sector?
The pharmaceutical sector in the United States is currently experiencing dynamic shifts driven by several global factors. One significant driver is the increasing demand for innovative therapies, particularly in areas such as oncology, immunology, and rare diseases. This demand is compelling companies to invest heavily in research and development (R&D), enhancing their product pipelines. Additionally, the COVID-19 pandemic has accelerated the adoption of digital health solutions, such as telemedicine and remote patient monitoring, which are becoming integral in drug distribution and patient engagement strategies.
For international B2B buyers, particularly those from Africa, South America, the Middle East, and Europe, understanding these trends is crucial. The rise of digital transformation in the pharmaceutical supply chain is enabling more efficient sourcing and procurement processes. Technologies such as artificial intelligence (AI) and blockchain are being utilized to enhance transparency and traceability in sourcing, which is vital for compliance with stringent regulatory requirements. Furthermore, partnerships between pharmaceutical companies and technology firms are becoming more common, allowing for innovative solutions that can streamline operations and reduce costs.
Emerging markets are also experiencing shifts, with many pharmaceutical companies looking to expand their presence in these regions. This trend presents opportunities for international buyers to engage with U.S. pharmaceutical firms seeking to enter or strengthen their foothold in new markets. Understanding local regulations and market needs will be key for effective collaboration.
How Are Sustainability and Ethical Sourcing Being Integrated into the Pharmaceutical Supply Chain?
In recent years, sustainability has become a critical focus for pharmaceutical companies, driven by both regulatory pressures and consumer demand for ethical practices. The environmental impact of pharmaceutical manufacturing is significant, with energy consumption, waste generation, and resource depletion being key concerns. Companies are increasingly adopting sustainable practices, such as utilizing renewable energy sources and implementing waste reduction strategies.
Ethical sourcing is also gaining traction, with companies striving to create transparent supply chains. This involves ensuring that raw materials are sourced responsibly and that suppliers adhere to labor and environmental standards. For international B2B buyers, partnering with U.S. pharmaceutical companies that prioritize sustainability can enhance their corporate social responsibility (CSR) initiatives and improve brand reputation.
Moreover, many pharmaceutical companies are pursuing ‘green’ certifications, such as ISO 14001, which focuses on effective environmental management systems. These certifications not only demonstrate a commitment to sustainability but can also facilitate access to new markets where environmentally conscious practices are valued. Buyers should actively seek out suppliers with established sustainability policies to mitigate risks and align with global sustainability goals.
What Is the Brief Evolution of the Pharmaceutical Industry in the U.S.?
The U.S. pharmaceutical industry has evolved significantly over the past century, transitioning from small-scale, localized production to a global powerhouse characterized by advanced research and innovation. The early 20th century marked the rise of major pharmaceutical companies, driven by breakthroughs in chemistry and biology. The establishment of the Food and Drug Administration (FDA) in 1906 set the stage for regulatory oversight, ensuring drug safety and efficacy.
By the mid-20th century, the industry experienced rapid growth, fueled by the introduction of antibiotics and vaccines. The 1980s and 1990s saw the advent of biotechnology, leading to the development of biologics and personalized medicine. Today, the industry is at the forefront of cutting-edge technologies, including gene therapy and advanced drug delivery systems. This evolution underscores the importance of innovation and adaptability in the pharmaceutical sector, providing valuable lessons for international B2B buyers seeking reliable partnerships. Understanding this historical context can help buyers appreciate the complexities of the industry and make informed sourcing decisions.
Frequently Asked Questions (FAQs) for B2B Buyers of list of pharmaceutical companies in usa
-
1. How can I ensure the reliability of pharmaceutical suppliers in the USA?
To ensure the reliability of pharmaceutical suppliers, conduct thorough due diligence. Verify their licenses and certifications, check for compliance with FDA regulations, and review their quality assurance processes. Request references from other international buyers and assess their operational history, including any past recalls or compliance issues. Additionally, consider third-party audits and certifications, which can provide insights into their manufacturing practices and product quality. -
2. What are the typical payment terms when sourcing pharmaceuticals from US companies?
Payment terms can vary widely among pharmaceutical suppliers. Common practices include net 30 or net 60 days, requiring payment within 30 or 60 days of invoice receipt. Some suppliers may request upfront payments or deposits, especially for custom formulations or large orders. Always negotiate terms before finalizing any agreements and consider using secure payment methods to protect your transaction. It’s also prudent to understand any currency conversion implications if you’re dealing with international suppliers. -
3. What minimum order quantities (MOQs) should I expect from US pharmaceutical companies?
Minimum order quantities can differ significantly depending on the pharmaceutical product and the supplier. Typically, MOQs for generic drugs can be lower, while branded medications may have higher thresholds. For custom formulations, suppliers may establish specific MOQs based on production costs and logistics. Always inquire about MOQs during initial discussions and assess whether they align with your business needs to avoid overcommitting resources. -
4. How do I verify the quality assurance processes of pharmaceutical companies in the USA?
To verify the quality assurance processes, request documentation detailing the supplier’s quality management system, including compliance with Good Manufacturing Practices (GMP). Ask for certificates of analysis for products and any relevant FDA inspection reports. Engaging third-party auditing services can also help assess the quality control measures in place. Regular communication with suppliers about their QA processes is essential to ensure that they meet international standards. -
5. What logistics considerations should I keep in mind when importing pharmaceuticals from the USA?
When importing pharmaceuticals, consider factors such as shipping methods, customs regulations, and temperature-sensitive storage requirements. Ensure that the supplier can provide appropriate packaging and shipping solutions to maintain product integrity during transit. Familiarize yourself with import duties and taxes in your country, as these can impact total costs. Establishing a reliable logistics partner can streamline the import process and help navigate any potential challenges. -
6. How can I customize pharmaceutical products to meet my market’s needs?
To customize pharmaceutical products, engage in direct discussions with suppliers about your specific requirements, such as formulation changes, packaging, or branding. Many suppliers offer services for custom formulations, but this may come with additional costs and MOQs. Ensure that any modifications comply with local regulations in your market, and consider conducting market research to validate the demand for customized products before proceeding. -
7. What regulatory challenges should I be aware of when sourcing pharmaceuticals from the USA?
International buyers must navigate various regulatory challenges, including compliance with both US and destination country regulations. This often involves understanding import permits, labeling requirements, and product registration processes. It’s crucial to collaborate with suppliers who are knowledgeable about these regulations and can assist in ensuring compliance. Additionally, consider consulting with legal or regulatory experts to mitigate risks associated with international pharmaceutical trade. -
8. How do I establish a long-term partnership with a pharmaceutical supplier in the USA?
To establish a long-term partnership, prioritize open communication and transparency. Regularly engage with suppliers to discuss performance, address any issues, and explore opportunities for collaboration. Building trust through consistent ordering and timely payments can foster a strong relationship. Additionally, consider signing long-term contracts that benefit both parties, ensuring stability and predictability in pricing and supply.
Important Disclaimer & Terms of Use
⚠️ Important Disclaimer
The information provided in this guide, including content regarding manufacturers, technical specifications, and market analysis, is for informational and educational purposes only. It does not constitute professional procurement advice, financial advice, or legal advice.
While we have made every effort to ensure the accuracy and timeliness of the information, we are not responsible for any errors, omissions, or outdated information. Market conditions, company details, and technical standards are subject to change.
B2B buyers must conduct their own independent and thorough due diligence before making any purchasing decisions. This includes contacting suppliers directly, verifying certifications, requesting samples, and seeking professional consultation. The risk of relying on any information in this guide is borne solely by the reader.
Strategic Sourcing Conclusion and Outlook for list of pharmaceutical companies in usa
In the ever-evolving landscape of the pharmaceutical industry, strategic sourcing has emerged as a critical component for international B2B buyers. By leveraging the extensive offerings of leading pharmaceutical companies in the USA, businesses can access innovative solutions, competitive pricing, and reliable supply chains. Key players such as Johnson & Johnson, Pfizer, and AbbVie not only dominate the market by capitalization but also provide diverse product portfolios that cater to various therapeutic needs.
For buyers in regions like Africa, South America, the Middle East, and Europe, establishing partnerships with these companies can lead to improved healthcare outcomes and enhanced market competitiveness. Understanding the nuances of the U.S. pharmaceutical market—such as regulatory frameworks and distribution channels—will empower buyers to make informed decisions.
Looking ahead, the demand for pharmaceutical products will only increase, driven by advancements in technology and healthcare. Now is the time for international buyers to engage with U.S. pharmaceutical companies, fostering relationships that will not only meet immediate needs but also pave the way for future growth and collaboration. Embrace the opportunities that strategic sourcing presents, and position your business for success in the global marketplace.