Top 13 Companies Tracking COVID Cases in the USA
Are you struggling to find reliable suppliers in the aftermath of COVID-19? You’re not alone! The pandemic shook the manufacturing landscape, making it crucial to partner with top-tier factories that prioritize safety and quality. In this article, we’ll dive into the top 30 COVID cases among U.S. manufacturers, helping you understand who stands out in this challenging environment.
Discover the benefits of choosing a factory that not only meets your production needs but also ensures a safe working environment for everyone. Ready to make informed decisions that will boost your business? Let’s explore the key players in the industry and how they can help you thrive in a post-pandemic world. Keep reading to find your ideal manufacturing partner!
Top 13 Covid Cases Usa Manufacturers
CDC – COVID Data Tracking Solutions
USA Today – News and Information Solutions
USAFacts – Real-Time COVID-19 Data Solutions
Domain: usafacts.org
Registered: 2012 ( 13 years )
Introduction: USAFacts provides comprehensive, real-time data on COVID-19 cases and deaths across all 50 states in the United States.
Today – COVID Variant Insights and Vaccination Guidance
CDC – COVID-19 Data Analysis Services
Domain: cdc.gov
Registered: 1997 ( 28 years )
Introduction: COVID-19 data collection, analysis, and dissemination services provided by the National Center for Health Statistics (NCHS).
Johns Hopkins University – COVID-19 Data Tracking Solutions
Domain: coronavirus.jhu.edu
Registered: 1987 ( 38 years )
Introduction: Johns Hopkins University provides comprehensive tracking and analysis of COVID-19 data globally, including case counts, vaccination rates, and hospitalization trends.
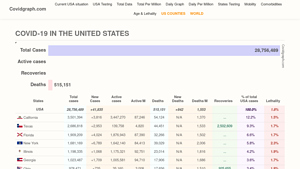
Covidgraph – COVID-19 Data Visualization Platform
Domain: covidgraph.com
Registered: 2020 ( 5 years )
Introduction: COVID-19 data visualization and analytics platform providing statistics on cases, deaths, recoveries, and testing across the United States.
Dailydot – News and Insights on Current Events
Nypost – COVID-19 Impact Reporting
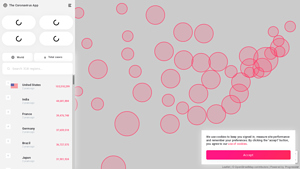
Coronavirus – COVID-19 Live Tracking Solutions
Domain: coronavirus.app
Registration year: Not available
Introduction: The Coronavirus App provides live tracking of COVID-19 cases in the United States and worldwide.
Statista – COVID-19 Vaccine Data Insights
Domain: statista.com
Registered: 2005 ( 20 years )
Introduction: COVID-19 vaccines approved for distribution and administration in the United States.
NPR – Coronavirus Tracking Platform
Domain: npr.org
Registered: 1993 ( 32 years )
Introduction: NPR provides a platform for tracking the spread of the coronavirus in the U.S. through charts and maps that display cases and deaths.
Economictimes – COVID-19 Insights and Analysis
Domain: economictimes.indiatimes.com
Registered: 1996 ( 29 years )
Introduction: Information not available.
Category Information
The category of “COVID cases in the USA” encompasses the tracking, reporting, and analysis of confirmed COVID-19 infections across the United States. This includes data on new cases, hospitalizations, recoveries, and fatalities, which are crucial for understanding the pandemic’s trajectory. Public health agencies, such as the Centers for Disease Control and Prevention (CDC) and state health departments, regularly update this information to inform the public and guide policy decisions.
The significance of monitoring COVID cases lies in its impact on public health strategies, resource allocation, and community awareness. Accurate data helps authorities implement measures such as lockdowns, vaccination campaigns, and travel restrictions, ultimately aimed at controlling the spread of the virus. Furthermore, this information is vital for researchers studying the virus’s behavior and effects, enabling them to develop effective treatments and preventive measures.
Application Information
Products and services related to “COVID cases USA” are primarily utilized in public health, research, and technology sectors. In public health, health departments and organizations track and analyze COVID case data to inform policies, allocate resources, and implement measures to curb the spread of the virus. This includes reporting systems, dashboards, and mobile applications that provide real-time updates and alerts to the public.
In research and academia, data on COVID cases is essential for epidemiological studies, vaccine development, and understanding the virus’s impact on different demographics. Researchers use this data to model infection trends and evaluate the effectiveness of interventions. Additionally, businesses leverage this information to make informed decisions about workplace safety, employee health policies, and operational adjustments in response to local COVID conditions. Overall, the integration of COVID case data across these areas supports informed decision-making and public health strategies.
Production Process Information
The production process for COVID-related products and services, such as tests, vaccines, and personal protective equipment (PPE), involves several key stages. First, there is research and development (R&D), where scientists and experts study the virus and develop effective solutions. This stage is crucial for ensuring that the products are safe and effective for public use. Once a product is developed, the next step is manufacturing.
This involves creating the product at scale, which can include assembling test kits, producing vaccines, or fabricating masks. Quality control is essential during this phase to ensure that every item meets health and safety standards. Finally, there is the distribution phase, where products are shipped to healthcare facilities, pharmacies, and other outlets. This ensures that they are readily available for testing and protection against COVID-19.
Throughout the process, clear communication with health authorities and the public is vital to keep everyone informed and safe.
Frequently Asked Questions (FAQs)
What should I look for in a COVID-19 test kit manufacturer?
When searching for a COVID-19 test kit manufacturer, it’s essential to consider several factors. First, check if the manufacturer is FDA-approved or has received Emergency Use Authorization (EUA) for their products. This ensures that the tests meet safety and efficacy standards. Additionally, look for manufacturers with a good track record, positive reviews, and transparent communication. It’s also helpful to inquire about their production capacity and supply chain reliability to ensure they can meet your demands.
How can I verify the quality of COVID-19 testing supplies?
To verify the quality of COVID-19 testing supplies, start by requesting product specifications and certificates from the manufacturer. Look for third-party testing results and compliance with relevant standards, such as ISO certifications. If possible, ask for samples to conduct your own evaluations or to have them tested by an independent laboratory. Engaging with other customers or reading testimonials can also provide insights into the reliability and performance of the products.
What are the typical lead times for ordering COVID-19 supplies?
Lead times for ordering COVID-19 supplies can vary widely depending on the manufacturer and the type of products you need. Generally, you can expect anywhere from a few days to several weeks for delivery. It’s a good practice to ask the manufacturer directly about their current lead times, as these can be influenced by demand, inventory levels, and shipping logistics. Planning ahead and placing orders early can help mitigate any potential delays.
Are there minimum order quantities I should be aware of?
Yes, many manufacturers have minimum order quantities (MOQs) for COVID-19 supplies. These MOQs can vary based on the type of product and the manufacturer’s policies. Some may require you to order a certain number of test kits or other supplies to fulfill your order. It’s important to clarify this with the manufacturer before placing an order to ensure that their MOQ aligns with your needs and budget.
What should I do if I encounter issues with a supplier?
If you encounter issues with a supplier, the first step is to communicate directly with them. Clearly outline your concerns and see if they can provide a resolution. Most reputable manufacturers will strive to address any problems promptly. If the issue persists, consider reviewing your contract or agreement for any terms related to disputes. You may also want to explore alternative suppliers if the situation cannot be resolved satisfactorily. Keeping records of all communications can be helpful if you need to escalate the matter.